Lịch sử
mua hàng
Glucosamine is an amino-monosaccharide synthesized from glucose and found in most body tissues, particularly in connective tissues and cartilage. It is naturally produced in the body and plays a crucial role in maintaining joint health. Glucosamine is commonly used to support the treatment of knee osteoarthritis and mild to moderate degenerative joint diseases. Among its forms, Glucosamine Sulfate is the most well-supported by clinical research, known for its high safety and effectiveness.
However, natural glucosamine levels decrease with age. Cartilage begins to break down over time, making movement more difficult and painful. Numerous clinical studies on glucosamine supplementation over the years have demonstrated its benefits:

Glucosamine is traditionally extracted from the shells of shellfish such as shrimp and crabs. However, the production process of glucosamine from shellfish consumes a significant amount of chemicals and energy while also generating excessive hazardous wastewater that harms humans, animals, and the environment. Additionally, shellfish-derived glucosamine is unsuitable for individuals with allergies, vegetarians, or those following religious dietary restrictions.
GlucosaGreen®, developed by TSI Group Ltd., is a sustainable plant-based alternative that addresses these concerns. Unlike traditional glucosamine, GlucosaGreen® is produced through a direct fermentation process, significantly reducing waste and carbon emissions while maintaining the same bioavailability.
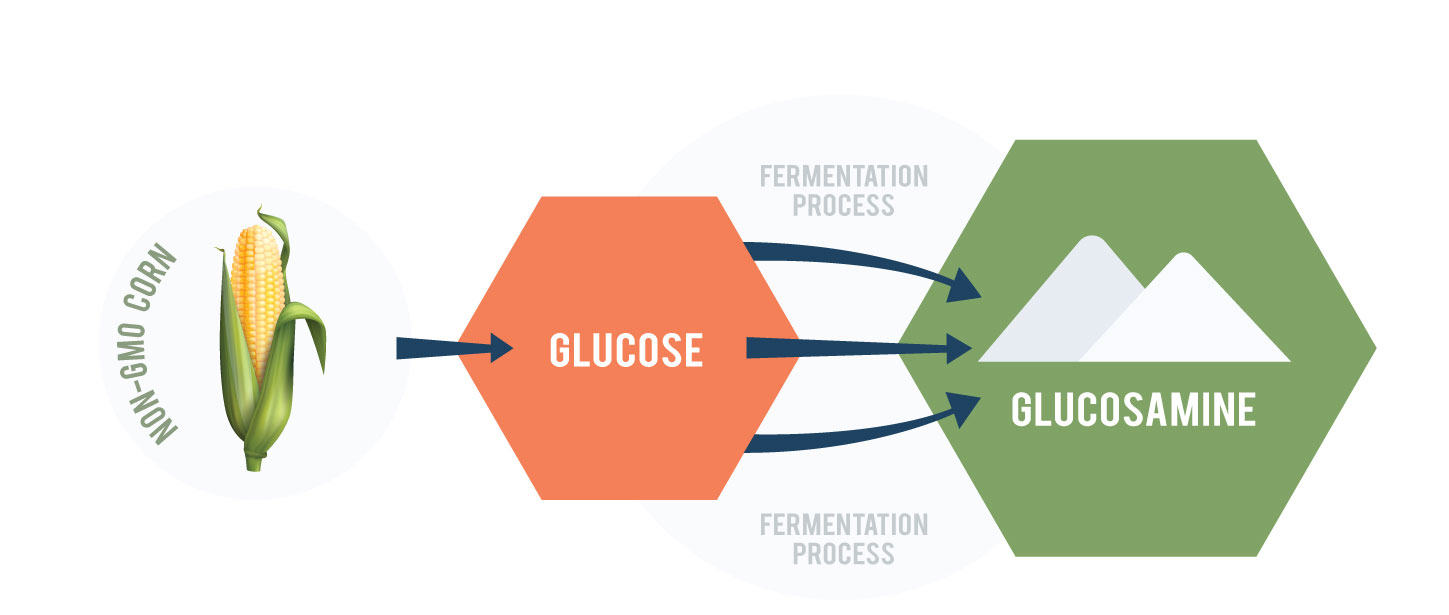
Launched by TSI in 2016, GlucosaGreen® is the world’s first commercially produced sustainable glucosamine, manufactured using a patented direct fermentation technology. Made from non-GMO corn, GlucosaGreen® has the same bioavailability as all other glucosamine sources while eliminating the environmental impact of shellfish-derived chitin production. It also boasts impressive environmental achievements:
Saves water
Shellfish glucosamine manufacturing requires 480 – 500 metric tons of clean water to make 1 metric ton of glucosamine HCI. GlucosaGreen® only requires 2 metric tons of clean water to produce the same amount of glucosamine. That’s 99.9% less water!
Reduce solid waste
Producing 1 metric ton of traditional shellfish glucosamine requires 30 – 40 metric tons of shellfish shells, and creates 5 – 6 metric tons of solid waste requiring landfill. GlucosaGreen® is shellfish free and produces significantly less solid waste.
Produces less harmful gases
The GlucosaGreen® manufacturing process requires significantly less hazardous chemicals, reducing the air pollution created in producing these chemicals that go into making shellfish-derived glucosamine.
This product is also suitable for vegetarians, kosher, Halal, and individuals allergic to shellfish.
GlucosaGreen® has many certifications and high quality standards, including:
Verified by laboratory testing to demonstrate bioequivalence to all forms of shellfish glucosamine
Manufactured to the highest cGMP standards
Non-GMO, shellfish-free and allergen-free
USP, JP and EP Compendial grades available
Available in all glucosamine salt forms
Available in ingredient and finished dosage form
GRAS affirmed
EU Novel food approval
GlucosaGreen® has recently obtained the NSF Carbon Footprint Certification and ISCC PLUS Sustainability Certification, as well as Non-GMO Certification and Vegan Certification (V-Label Criteria by SGS).
Will Ji, President of the TSI Optimized Ingredients Product Line, explained that TSI aims to strongly state that innovation is still happening in the glucosamine sector.
"We saw the need for a different option, and we are doing something better. These certifications create a cohesive bundle to reinforce our quality, standards and processes."
GlucosaGreen® is available in various salt forms, including Glucosamine Sulfate, Glucosamine Hydrochloride, and Glucosamine N-Acetyl-D, meeting diverse needs. The product comes in multiple forms such as powder, direct compression granules, capsules, and chewable tablets. With consumers increasingly prioritizing eco-friendly products, GlucosaGreen® helps brands enhance their reputation and meet sustainability standards. It also enables businesses to comply with strict regulations on sustainability, clean labeling, and allergen-free products
1) Ostojic et al. Glucosamine administration in athletes: effects on recovery of acute knee injury. Res Sports Med 15:113-124, 2007.
2) Yoshimura et al. Evaluation of the effect of glucosamine administration on biomarkers for cartilage and bone metabolism in soccer players. Int J Molec Med 24:487-494, 2009.
3) Runhaar et al. The role of diet and exercise and of glucosamine sulfate in the prevention of knee osteoarthritis: Further results from the Prevention of knee Osteoarthritis in Overweight Females (PROOF) study Semin Arthritis Rheum 45: S42-S48, 2016
4) Tomonaga et al. Evaluation of the effect of N‑acetyl‑glucosamine administration on biomarkers for cartilage metabolism in healthy individuals without symptoms of arthritis: A randomized double‑blind placebo‑controlled clinical study. Exp Ther Med 12: 1481-1489, 2016
Glucosagreen.com